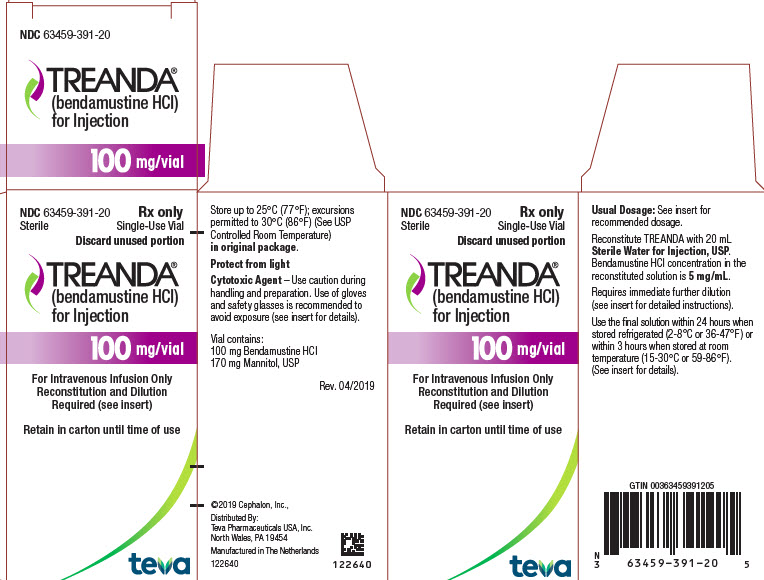
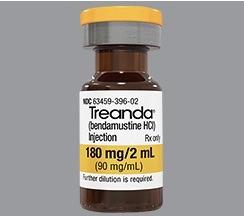
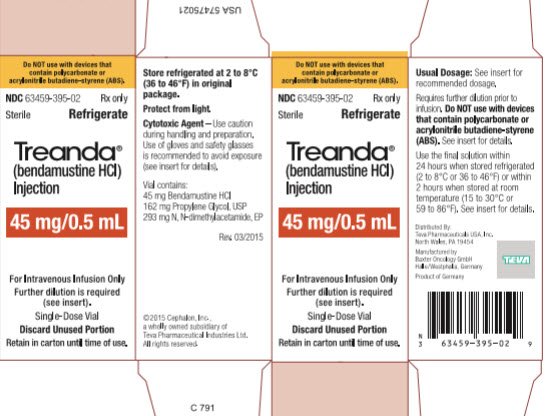
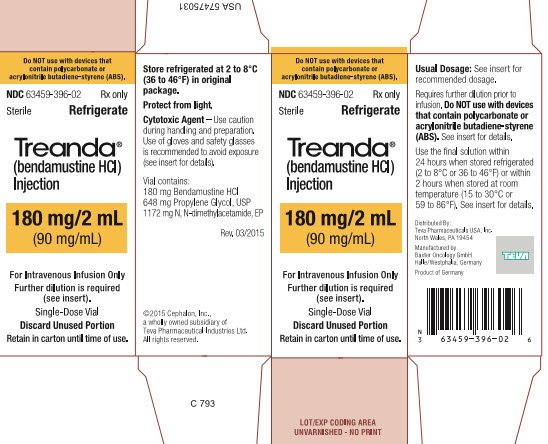
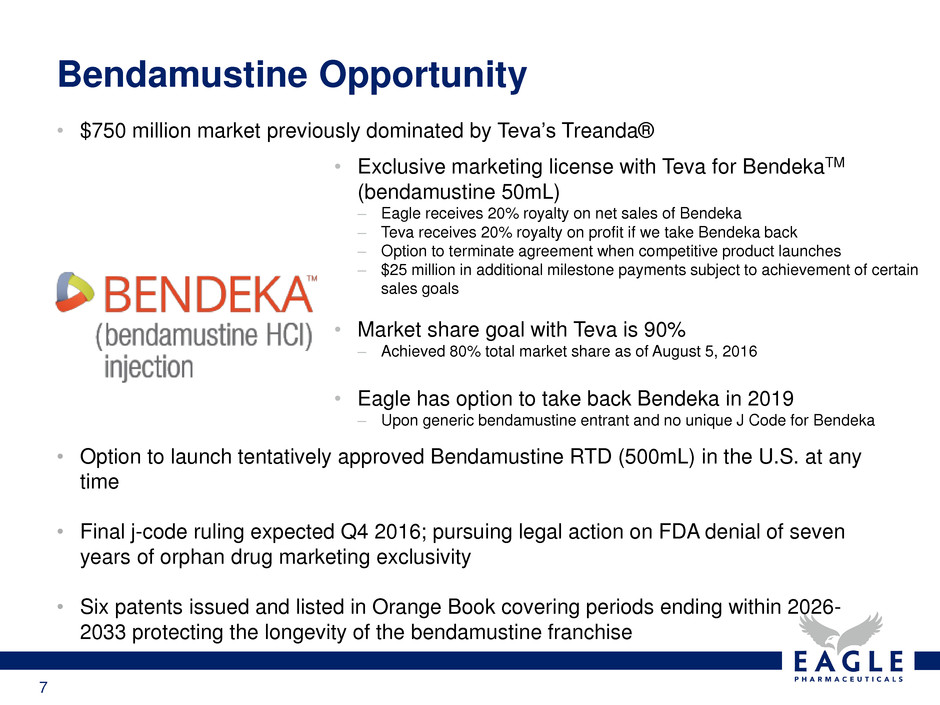
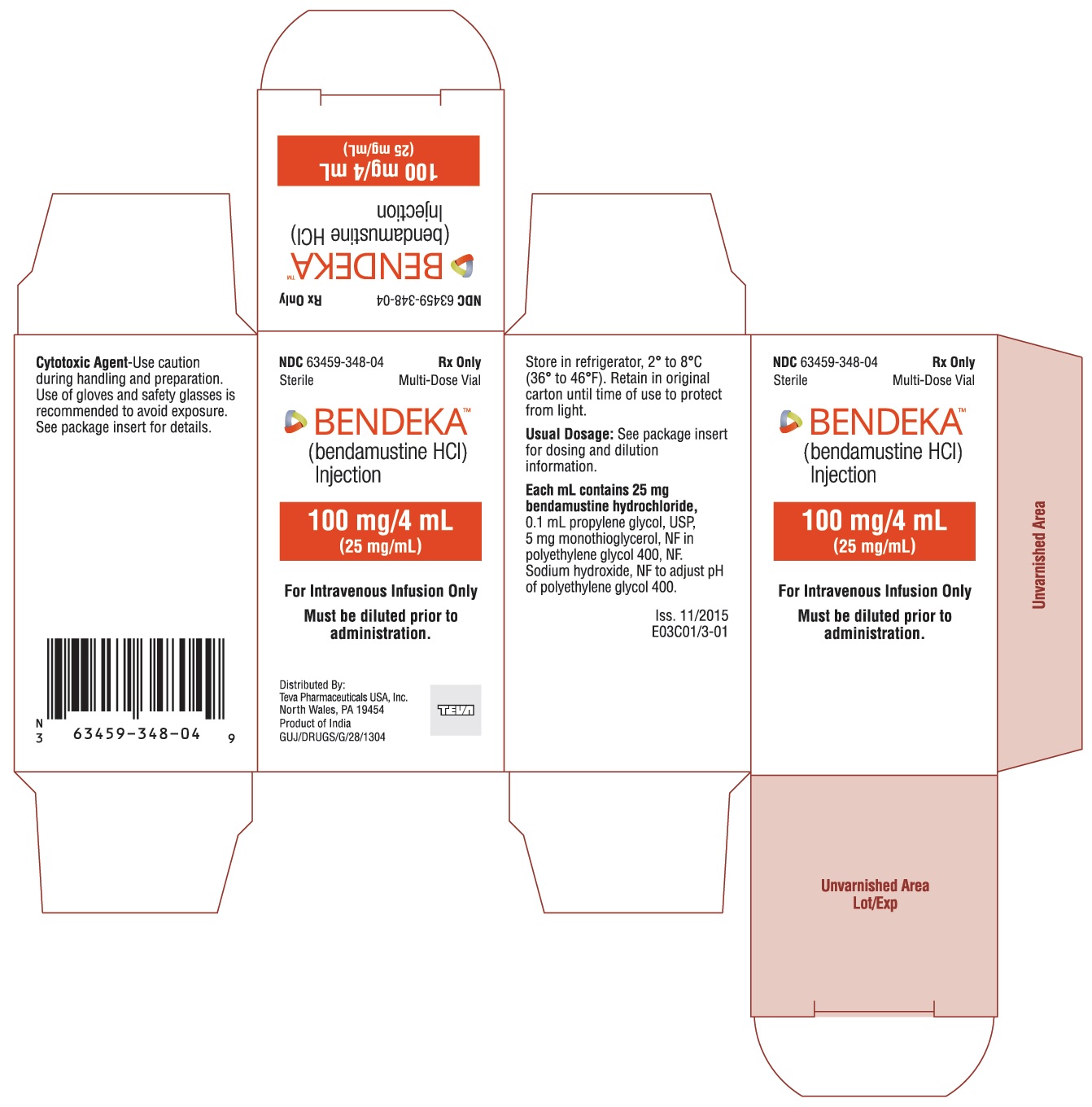
These highlights do not include all the information needed to use BENDEKA safely and effectively. See full prescribing information for BENDEKA. BENDEKA® (bendamustine hydrochloride injection), for intravenous use Initial U.S. Approval: 2008
CMS Determines that Existing J-Code J9033 Adequately Describes BENDEKA ( bendamustine hydrochloride) Injection
Teva Announces Favorable Court Ruling in TREANDA® (bendamustine hydrochloride) Patent Infringement Litigation

These highlights do not include all the information needed to use BENDEKA safely and effectively. See full prescribing information for BENDEKA. BENDEKA® (bendamustine hydrochloride injection), for intravenous use Initial U.S. Approval: 2008