Sciencemadness Discussion Board - Synthesis of Phenylacetylcarbinol by Alkyne Hydration and Subsequent Enamine formation - Powered by XMB 1.9.11

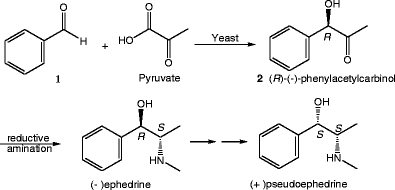
Biotransformation of benzaldehyde to L‐phenylacetylcarbinol (L‐PAC) by Torulaspora delbrueckii and conversion to ephedrine by microwave radiation | Semantic Scholar

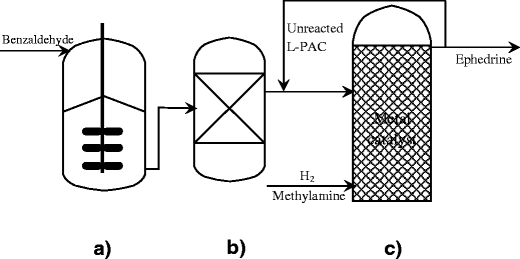
Investigation of the l-phenylacetylcarbinol process to substituted benzaldehydes of interest - ScienceDirect

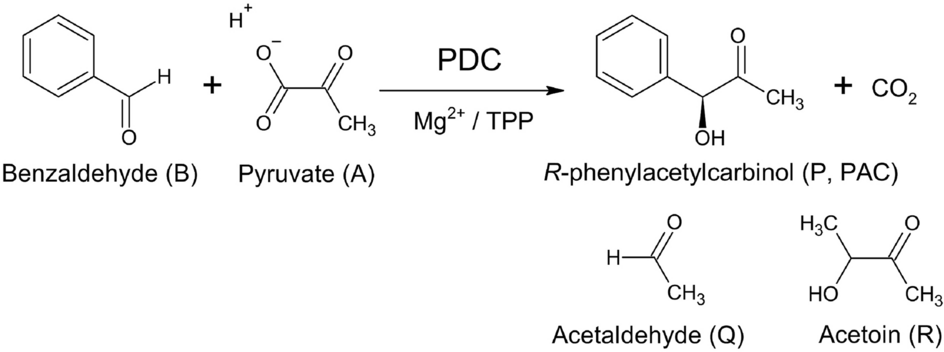
a–c Reactions catalyzed by pyruvate decarboxylase (PDC). a Pyruvate... | Download Scientific Diagram
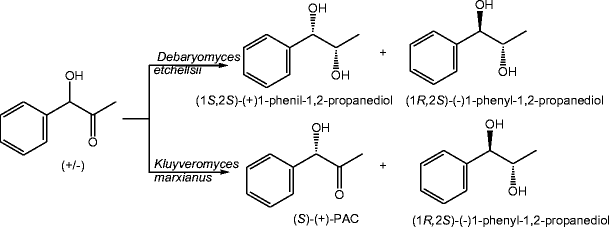
Potential of some yeast strains in the stereoselective synthesis of (R)-(−)- phenylacetylcarbinol and (S)-(+)-phenylacetylcarbinol and their reduced 1,2-dialcohol derivatives | SpringerLink

Investigation of the l-phenylacetylcarbinol process to substituted benzaldehydes of interest - ScienceDirect

Improvement of the yeast based (R)-phenylacetylcarbinol production process via reduction of by-product formation - ScienceDirect
![PDF] The yeast mediated synthesis of the l-ephedrine precursor, l- phenylacetylcarbinol, in an organic solvent | Semantic Scholar PDF] The yeast mediated synthesis of the l-ephedrine precursor, l- phenylacetylcarbinol, in an organic solvent | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/96025f6077bf82ffa0b924f17d008ebacae4a94e/22-Figure1.1-1.png)
PDF] The yeast mediated synthesis of the l-ephedrine precursor, l- phenylacetylcarbinol, in an organic solvent | Semantic Scholar
Validation of mathematical model with phosphate activation effect by batch (R)-phenylacetylcarbinol biotransformation process utilizing Candida tropicalis pyruvate decarboxylase in phosphate buffer | Scientific Reports

Figure 1 from Screening of Yeasts for Cell-Free Production of (R)- Phenylacetylcarbinol in a Shake Flask Condition | Semantic Scholar